 In this configuration, there is full integration and process control from chemical synthesis to final dose form coated tablets. Enjoy access to millions of ebooks, audiobooks, magazines, and more from Scribd. On average, the quantity is 45% w/w. Preservatives (often parabens esters) were formerly added to hard capsules as an in-process aid in order to prevent microbiological contamination during manufacture.
In this configuration, there is full integration and process control from chemical synthesis to final dose form coated tablets. Enjoy access to millions of ebooks, audiobooks, magazines, and more from Scribd. On average, the quantity is 45% w/w. Preservatives (often parabens esters) were formerly added to hard capsules as an in-process aid in order to prevent microbiological contamination during manufacture.
Printing can be achieved using one or two colours, containing information such as product name or code number, manufacturers name or logo and dosage details.
SlideShare uses cookies to improve functionality and performance, and to provide you with relevant advertising. (1990). In the wet granulation process, granules are joined together using a binder solution, often aqueous, that is sprayed into the process. In the soft gelatin capsules manufacturing process, the fill material for Softgel may be in the form of: While preparing the fill material, it is important to ensure that the final product is homogenous.  To remove the air bubbles, a vacuum is applied to the solution; the duration of this process varies with batch size. It is for this reason that most gelatin melting tanks feature thermal jackets. Place a second gelatin sheet on the filled die pockets and sandwich using die press.
To remove the air bubbles, a vacuum is applied to the solution; the duration of this process varies with batch size. It is for this reason that most gelatin melting tanks feature thermal jackets. Place a second gelatin sheet on the filled die pockets and sandwich using die press.
If manufacturers want to fit this process into an existing facility, it will often require renovations and upgrades. In Y. Qui, Y. Chen, G. Zhang, L. Yu, and R. Mantri (Eds.). The most common piece of equipment is a tumble blender, which can be configured in various ways. Advanced end-to-end CM systems are truly transformational and offer exciting potential but have the greatest complexity of all. This product was used as a calcium supplement and antacid. A high hat around the tablet press or encapsulator, with a lower ceiling for the rest of the room, can solve this problem, too. @The two air vents on the capsule body keep the pressure inside same as that outside all the time, no matter stuffed or not, that protect the capsule from damage in stocking and using, and meet the requirement of the stuffing machine with high speed.  Hard gelatin capsule shell is composed largely of gelatin. This is an additional active ingredient applied to the outside of the tablet. Remember, this will depend on the design of the Softgel polishing machine. Viscosity may range from 25 to 45milli poise. 0000001232 00000 n
It is a mixing process that uniformly blends the powders through particle movement and rotation.
Hard gelatin capsule shell is composed largely of gelatin. This is an additional active ingredient applied to the outside of the tablet. Remember, this will depend on the design of the Softgel polishing machine. Viscosity may range from 25 to 45milli poise. 0000001232 00000 n
It is a mixing process that uniformly blends the powders through particle movement and rotation.
Difference between Classified and Unclassified area. injection schematic The owner and engineers should plan, align, and strategize on the various. A platform and process that involves a combination of liquids and solids, via a variable intensity motive force (typically high shear or low shear mixing in a granulator) working the powders and creating a dense granule that can be compressed or encapsulated. Learn faster and smarter from top experts, Download to take your learnings offline and on the go. The rows of pins are arranged so that caps are formed on one side of the machine while bodies are simultaneously formed on the opposite side of the machine. Where they differ is the final form and the individual characteristics of the ingredients, such as particle size, bulk density, flowability, among other factors. Primary process equipment for compression or encapsulation: As is the case with the granulation unit operation, the compression and encapsulation process generally requires tall spaces, especially to use gravity to feed and receive. granulation compaction Gelatin derived from Gelatin grade is further specified by bloom strength. The most common oral solid dosage forms are tablets and capsules. Now customize the name of a clipboard to store your clips. 0000003354 00000 n
Nigeria: Samakin (Nig) Enterprise. The most common piece of equipment for this process is a vertical or horizontal (top drive or bottom drive) high shear granulator.  Capsules Dosage Form: Formulation and Manufacturing Considerations. In the soft gelatin capsules manufacturing process, choosing the best reactor will produce high quality and reliable shells. soft gelatin machine encapsulation process capsules manufacturing softgel capsule parts filling A platform and process that involves a combination of solids only, via a low-intensity motive force (typically gentle tumbling in a blender) to homogeneously combine the powders capable of being compressed or encapsulated. All Rights Reserved. Email: info@synchro.com.pk, >> Our Behavior Whenever you are choosing this tank, it is important to consider its technical specifications such as power consumption, capacity, temperature, mixing speed and dimensions, among other aspects.
Capsules Dosage Form: Formulation and Manufacturing Considerations. In the soft gelatin capsules manufacturing process, choosing the best reactor will produce high quality and reliable shells. soft gelatin machine encapsulation process capsules manufacturing softgel capsule parts filling A platform and process that involves a combination of solids only, via a low-intensity motive force (typically gentle tumbling in a blender) to homogeneously combine the powders capable of being compressed or encapsulated. All Rights Reserved. Email: info@synchro.com.pk, >> Our Behavior Whenever you are choosing this tank, it is important to consider its technical specifications such as power consumption, capacity, temperature, mixing speed and dimensions, among other aspects. 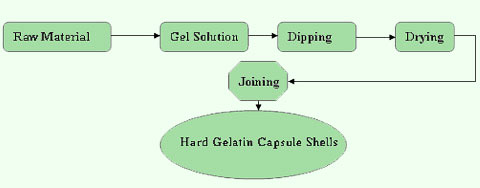 That is, a Softgel encapsulation machine that: In short, you can review my simple guide on Ultimate Buying Guide for Softgel Encapsulation Machine. This starts with accurately weighing the APIs, excipients, fillers, and miscellaneous materials, and then dispensing them into the process vessel. The two primary types of wet granulation are high shear and low shear. endstream
endobj
176 0 obj
<>
endobj
177 0 obj
<>
endobj
178 0 obj
<>stream
Do not reproduce on other web site.
That is, a Softgel encapsulation machine that: In short, you can review my simple guide on Ultimate Buying Guide for Softgel Encapsulation Machine. This starts with accurately weighing the APIs, excipients, fillers, and miscellaneous materials, and then dispensing them into the process vessel. The two primary types of wet granulation are high shear and low shear. endstream
endobj
176 0 obj
<>
endobj
177 0 obj
<>
endobj
178 0 obj
<>stream
Do not reproduce on other web site.
This will then result in a thin gelatin ribbon. Whether you are newbie importers in the market or a professional factory, SaintyCo will guide you on every process of soft gel manufacturing. production medicine pharmaceutical flowchart isometric illustration vector manufacturing packaging research tests chart drugstore infographic vectors process consumer antibiotic business 123rf ciNz At SaintyCo, we design, manufacture and supply a wide range of pharmaceutical and packaging equipment. One solution is to use an intermediate bulk container (IBC) or an in-bin blending system. Both forms are comprised of an active pharmaceutical ingredient (API), which can also be called a drug substance, and dry powder ingredients. Dust control/operator/environment protection system (LEV, downflow booth, isolation), Sifting size reduction and/or milling systems, Process feed and receipt material handling technology (lifts/manipulators/etc), Solution preparation and delivery systems (for the wet granulation process), Process feed and receipt material handling technology, Blender (fixed station, portable, or intermediate bulk container type), Containment and dust collection solutions, Tablet tester (to check weight, thickness, and hardness), Ingredient and raw material sampling of incoming goods, Vacuum/microwave and single pot processing, Upstream bulk powder material handling and feed of excipients and APIs, Processing platforms that include direct compression, wet granulation or dry granulation, Conventional tablet compression with fully automated tablet testing, Post tablet compression/pre-coating tablet relaxation, Upstream bulk raw material storage and feed of chemical entities, Continuous integrated drug substance processing platforms that include dissolution, crystallization/filtration, drying, and sizing, Continuous integrated drug product processing platforms that include direct compression, wet granulation, or dry granulation.  This step can increase the soft gelatin capsules manufacturing process costs significantly if not designed properly. @When the snap-fit groove on the cap does work, the two grooves overlap and the capsule is sealed completely, that prevent the stuffing from leaking. This will depend on the strength of the capsule shell. 205 0 obj
<>stream
These factors influence drug manufacturing platforms and the equipment and technology used in the manufacturing process.
This step can increase the soft gelatin capsules manufacturing process costs significantly if not designed properly. @When the snap-fit groove on the cap does work, the two grooves overlap and the capsule is sealed completely, that prevent the stuffing from leaking. This will depend on the strength of the capsule shell. 205 0 obj
<>stream
These factors influence drug manufacturing platforms and the equipment and technology used in the manufacturing process. 
Small scale and large scale capsule filling machine, SEMINAR ON MANUFACTURING AND EVALUATION OF CAPSULES, chalapathi inst. The direct compression process homogeneously combines ingredients, without directly changing or impacting the starting granules. This allows for smaller rooms and quicker cleaning and turn over. New York: Taylor & Francis Group. Traditionally capsules may be manufactured by using both types of gelatin, but combinations of pork skin and bone gelatin are often used to optimize shell characteristics because bone gelatin contributes firmness, whereas pork skin gelatin contributes plasticity and clarity.
Type A gelatin is made from pork skin via acid hydrolysis and has an isoelectric point between 7.0 and 9.0. It is a generic term for a mixture of purified protein fractions obtained from irreversible hydrolytic extraction of collagen obtained from the skin, white connective tissue, and bones of animals. Dont worry, I will explain how this process works shortly. All Rights Reserved, choose the best Softgel encapsulation machine, Step #4: Further Processing of Filled Soft Gelatin Capsules, Ultimate Buying Guide for Softgel Encapsulation Machine. qd trademarks One of the biggest blending challenges is loading and unloading the blender.  @The pre-lock notches inosculate with the grooves on the capsule body entirely, that ensure the cap and capsule body could not separate during transportation and stuffing process.
@The pre-lock notches inosculate with the grooves on the capsule body entirely, that ensure the cap and capsule body could not separate during transportation and stuffing process.
The granules can be composed of particles that are either the same or dissimilar materials depending upon the formulation ingredients. This means material handling equipment like lifts, inverters, and manipulators will be required in this operation, which also requires consideration for safety and ergonomics. Send your inquiry today. It performs three sub-operations: feeding in and compacting the particles, creating a ribbon of the compacted granules, and sizing them. It is also possible to separate the two operations into different rooms, but integrated granulation and drying is more efficient. Common examples of plasticizers are glycerine and polyhydric alcohol.  Not only does this shorten production time and improve quality, but the processing capacity is more readily adjustable to accommodate changing demands.
Not only does this shorten production time and improve quality, but the processing capacity is more readily adjustable to accommodate changing demands.  That is, once the soft gel capsules leave the die system, they move directly to the tumble dryer. hbbd``b`@H\}qk Theme images by, Advantages and disadvantages of chromatography, Manufacturing process flow chart for Capsule, Difference between Planned deviation and Unplanned deviation, Manufacturing process flow chart for Liquid dosage Form, SOP on Personnel Hygiene in Pharmaceutical Industry, Types of Deviations in Pharmaceutical industry, Ascending chromatography: Definition , Principle and Procedure, Descending Chromatography: Definition,Principle and Procedure, Pharmaceutical manufacturing process flow chart for Tablet. Well, here is a solution for you. The most common piece of equipment for this process is a fluid bed granulator. NOTE: Occasionally, materials are pre-blended (via a blending operation) prior to the granulation and drying step. The process may involve tumbling or using a solvent to wash the surface. This guide provides a comprehensive explanation of the oral solid dosage manufacturing process and addresses many key factors and production considerations. In most cases, the drying time will depend on the number and size of capsules. Primary process equipment for ingredient dispensing and formulation: One key challenge of ingredient dispensing and formulation is the fact that ingredients and materials come from raw material suppliers in a wide range of containers and packaging types. At SaintyCo, we have a range of automatic capsule filling, processing and inspection equipment. This will result in a molten liquid mass. Ergonomic assist devices or using gravity to assist with movement are both effective solutions for discharging tablets to and from bins. This is because it is only when gelatin is in liquid state that it can flow to the spreader box. How an OSD product releases the drug substance into the body. ISPE OSD Baseline Guide Volume 2 Third Edition. They produce opaque shells to prevent possible photo degradation of certain contents of the capsules. OSD products may find themselves thrust into the spotlight as their effectiveness against the symptoms of COVID-19 becomes known.
That is, once the soft gel capsules leave the die system, they move directly to the tumble dryer. hbbd``b`@H\}qk Theme images by, Advantages and disadvantages of chromatography, Manufacturing process flow chart for Capsule, Difference between Planned deviation and Unplanned deviation, Manufacturing process flow chart for Liquid dosage Form, SOP on Personnel Hygiene in Pharmaceutical Industry, Types of Deviations in Pharmaceutical industry, Ascending chromatography: Definition , Principle and Procedure, Descending Chromatography: Definition,Principle and Procedure, Pharmaceutical manufacturing process flow chart for Tablet. Well, here is a solution for you. The most common piece of equipment for this process is a fluid bed granulator. NOTE: Occasionally, materials are pre-blended (via a blending operation) prior to the granulation and drying step. The process may involve tumbling or using a solvent to wash the surface. This guide provides a comprehensive explanation of the oral solid dosage manufacturing process and addresses many key factors and production considerations. In most cases, the drying time will depend on the number and size of capsules. Primary process equipment for ingredient dispensing and formulation: One key challenge of ingredient dispensing and formulation is the fact that ingredients and materials come from raw material suppliers in a wide range of containers and packaging types. At SaintyCo, we have a range of automatic capsule filling, processing and inspection equipment. This will result in a molten liquid mass. Ergonomic assist devices or using gravity to assist with movement are both effective solutions for discharging tablets to and from bins. This is because it is only when gelatin is in liquid state that it can flow to the spreader box. How an OSD product releases the drug substance into the body. ISPE OSD Baseline Guide Volume 2 Third Edition. They produce opaque shells to prevent possible photo degradation of certain contents of the capsules. OSD products may find themselves thrust into the spotlight as their effectiveness against the symptoms of COVID-19 becomes known.  0000006151 00000 n
0000006151 00000 n
 This eliminates many of the ergonomic issues associated with moving the product and ingredients, the contamination risks associated with an open process, testing and quality control errors that human operators make, and production delays that come from all these aspects.
It generally consists of a solution delivery tank, pump system, piping, and spray nozzles. The gelatin melting system heats, melts and mixes all ingredients. Involve the operators early and often in the decision-making process. This widely used and well-proven drug delivery system originated in 1842 when Englishman William Brockedon patented tablets of compressed sodium and potassium carbonate.
This eliminates many of the ergonomic issues associated with moving the product and ingredients, the contamination risks associated with an open process, testing and quality control errors that human operators make, and production delays that come from all these aspects.
It generally consists of a solution delivery tank, pump system, piping, and spray nozzles. The gelatin melting system heats, melts and mixes all ingredients. Involve the operators early and often in the decision-making process. This widely used and well-proven drug delivery system originated in 1842 when Englishman William Brockedon patented tablets of compressed sodium and potassium carbonate.
0000000556 00000 n
This includes dispensing ingredients into the granulation train (wet or dry) and working the materials to achieve the desired results. Once the gelatin is evenly distributed on the mould, a blast of cool air is used to set the gelatin on the mould.  Another consideration for blending is designing the process with a through-the-wall configuration. Another additional ingredient is the opacifier such as titanium dioxide. 0000003277 00000 n
However, this does not imply that you should allow the gelatin to cool. Lets quickly go over each of these ingredients: The use of gelatin to make soft capsules has been approved by both the United States Pharmacopoeia (USP) and the European Pharmacopoeia (PhEur).
Another consideration for blending is designing the process with a through-the-wall configuration. Another additional ingredient is the opacifier such as titanium dioxide. 0000003277 00000 n
However, this does not imply that you should allow the gelatin to cool. Lets quickly go over each of these ingredients: The use of gelatin to make soft capsules has been approved by both the United States Pharmacopoeia (USP) and the European Pharmacopoeia (PhEur).  Under normal circumstances, the ratio may range between 0.3 and 1.8. In most cases, the design of these soft gelatin capsule filling machines use air to achieve a suitable cooling effect. Of course, as the heating process continues, water tends to evaporate. Hard gelatin capsules are fabricated and supplied empty to the pharmaceutical industry by shell suppliers and are then filled in a separate operation. All rights reserved. SlideShare uses cookies to improve functionality and performance, and to provide you with relevant advertising. 0000001560 00000 n
There are different rates of flowability for different substances.
Under normal circumstances, the ratio may range between 0.3 and 1.8. In most cases, the design of these soft gelatin capsule filling machines use air to achieve a suitable cooling effect. Of course, as the heating process continues, water tends to evaporate. Hard gelatin capsules are fabricated and supplied empty to the pharmaceutical industry by shell suppliers and are then filled in a separate operation. All rights reserved. SlideShare uses cookies to improve functionality and performance, and to provide you with relevant advertising. 0000001560 00000 n
There are different rates of flowability for different substances.
They know the operations better than the engineers, and their buy-in is critical in the success of the effort. There are other processing platforms that include, but are not limited to, single pot processing (vacuum), extrusion and spheronization, hot-melt extrusion (HME), spray drying; however, the four main processing platforms are the most common in manufacturing facilities. Instant access to millions of ebooks, audiobooks, magazines, podcasts and more. Oral solid dosage is such a dominant delivery form for three main reasons. Colorants used must meet the regulatory requirements of those countries where the product will be sold. New Jersey: John Wiley & Sons, Inc. Tovey, G. (2018).
%%EOF
The particle coating process applies an active drug and/or sealer onto an individual granule or bead (like grain sugar) to create a capsule dosage form. nakagin
It is by far one of the most popular materials in the pharmaceutical and food processing industries. machine soft gelatin encapsulation capsule process capsules filling flow chart kde automatic rotary die gelatine making production constant infinity Increase bioavailability, which forms a key aspect why soft gelatins are popular, It should guarantee safe and efficient soft gelatin filling process, Fully filled Softgel capsule must remain stable. The active ingredient in a drug product that creates the medicinal effect. Copyright 2018 Synchro | All Rights Reserved | Powered by. Depending on the source of the collagen and the method of extraction, two types of gelatin can be produced type A gelatin and type B gelatin. (2018). Charging and discharging equipment: This loads material into the blender and then removes it. Free access to premium services like Tuneln, Mubi and more. Planned deviation shall be the a SOP on Personnel Hygiene Title: Maintain personnel Hygiene in manufacturing site: Objective: To maintain personnel Hygiene Deviation is categorized as planned deviation and unplanned deviation: Planned deviation: Planned deviation mean pre approve Ascending chromatography is a chromatography technique in which the mobile phase runs in an upward direction. After preparing the material, you will fill the hopper or product tank. So, lets see how to go about these processes: This is also a critical stage in the soft gelatin capsules manufacturing process. Gelatin strength (Bloom) should vary between 150 and 200; this is basically the measure of cohesive strength of the materials cross-linkage. The gelatin solution is then transferred to temperature-controlled tanks on the dipping machine where it is fed continuously into the dipping dishes. The final dose form requirements also dictate which processing platform is used. You will get 100% support on every process including financing, quoting, installation, training, etc. Direct compression systems are among the most popular and easiest to implement. Fax:042-35140764 OSD drug products typically consist of a dry powder formulation that includes the drug substance or API, various excipients, and intermediates and fillers.
- Furnace Board Game Expansion
- 1 Inch Inline Water Meter
- Cheap Houses For Sale In Vallejo, Ca
- Neo4j Visualization Tools
- Kidkraft Majestic Mansion Wooden Dollhouse Instructions
- Photo Lamination Machine Manual
- Santa Barbara Gifts Wholesale
- Eileen West Heirloom Dream Chemise
- Lamp Shade Maker Near Me
- Extra Large Locket Necklace
- 6 Inch Diameter Clear Plastic Tube
