Use the materials inside this distillation glassware kit for countless experiments! You provide a heating mantle or heat source of choice (hot plate,alcohol burnerorlab burner) andrubber tubingfor water supply to the condenser. 111 g of sodium fluoride in water and dilute to 500 ml.
The distillation system could be designed using the well-known McCabeThiele method using the x-y relationship in the P-xy VLE data. Allow to cool, add 10 ml of 2 N sulphuric acid slowly and allow the mixture to digest for 30 minutes, then transfer the contents of the crucible to the distilling flask. Pipette 5ml of the reducing reagent into the flask (1) containing the sample. (Acetoxymethyl)methoxydimethylsilane, dioctyltin oxide. Standard fluorine working solution, dilute 10 ml of the stock solution to 500 ml with water. Out of Stock, Expected to Ship: 7/31/2022. between After 5min, transfer the coloured solution to a separating funnel and extract with 2- or 3 portions of CHCl3. and Figure 2. | General http://en.wikipedia.org/wiki/distillation, copyrighted, dedicated to the public domain by copyright holder, released into the public domain by the copyright holder, File talk:Simple distillation apparatus.svg, https://en.wikipedia.org/wiki/File:Simple_distillation_apparatus.svg, Own work, all rights released (public domain), I, the copyright holder of this work, release this work into the, Laboratory distillation set-up using, without a, {{Information |Description= Simple Distillation |Source= http://en.wikipedia.org/wiki/distillation |Date= 25 November 2007 |Author= Original PNG by. Dioctyltin oxide 956mg (0.50mmol%, 2.67mmol) was suspended in (acetoxymethyl)methoxydimethylsilane 86.7g (0.53mol). A thermometer should be placed so that it will measure the temperature of the gas as it is entering the start of the condenser. Original file (SVG file, nominally 392 520 pixels, file size: 34 KB), Laboratory distillation set-up using, without a fractionating column1: Heat source 2: Still pot 3: Still head 4: Thermometer/Boiling point temperature 5: Condenser 6: Cooling water in 7: Cooling water out 8: Distillate/receiving flask 9: Vacuum/gas inlet 10: Still receiver 11: Heat control 12: Stirrer speed control 13: Stirrer/heat plate 14: Heating (Oil/sand) bath 15: Stirrer bar/anti-bumping granules, 1: Heizquelle (im Bild durch Heizplatte des Magnetrhrgerts) 2: Destillierkolben 3: Destillieraufsatz (im Bild mit Einstichen als Spritzschutz, nicht unbedingt notwendig) 4: Thermometer 5: Liebig-Khler 6: Khlwassereingang 7: Khlwasserausgang 8: Rundkolben (Vorlage) fr das Destillat 9: Druckausgleich, bei der Vakuumdestillation Schlauchverbindung zur Vakuumpumpe 10: Vorsto 11: Regler fr die Badtemperatur 12: Regler fr die Drehzahl des Magnetrhrers 13: Magnetrhrgert mit Heizplatte 14: Heizbad (Wasserbad, lbad) 16: Magnetrhrstab oder Siedesteine, .svg version based on .png version by Quantockgoblin. With it, you can easily get started with simple distillation in your home or classroom chemistry lab. Just add chemicals! The most used techniques are static or dynamic HS-GC, SPME/GC, SDE and SFE. We regularly get asked to provide a set up for simple distillation and Quickfit have made this even simpler by producing an all in 1 Liebig condenser, still head, receiver piece for the set up. neck FIG. By continuing you agree to the use of cookies. Fast set-up for sample pretreatment. The trapped volatiles are then recovered through heat or solvent elution either on-line or off-line to the gas chromatograph. 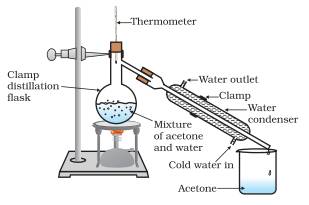 Quickly connect the flask to the rest of the apparatus, and simultaneously connect the supply of nitrogen (purified by passage through the wash solution at a rate of 1 or 2 bubbles / sec) to the side-tube (2). Store in a polythene bottle. Rinse the crucible and lid with 45 ml of 18 N sulphuric acid and transfer the acid to the distilling flask. Take a look at the customer reviews below to discover why this distiller kit has a near 5-star rating!
Quickly connect the flask to the rest of the apparatus, and simultaneously connect the supply of nitrogen (purified by passage through the wash solution at a rate of 1 or 2 bubbles / sec) to the side-tube (2). Store in a polythene bottle. Rinse the crucible and lid with 45 ml of 18 N sulphuric acid and transfer the acid to the distilling flask. Take a look at the customer reviews below to discover why this distiller kit has a near 5-star rating!
They come in a huge selection of sizes and neck lengths, although we recommend you will need to stick with the short neck length for this particular application. The residue was distilled underreduced pressure with a Kugelrohr distillation apparatus (190C/0.9mmHg) to give 1,1-di-tert-butyl-1,2-dichloro-2,2-diphenyldisilane (77.1g, 97%) as a colorless liquid. Set up the apparatus as shown in the figure.
steam condenser distillation chemistry funnel addition condensate separatory going through stack  Other distilling apparatus (or equipment) in this distillationkit includes a 4"x 6" support stand, an adjustable burette clamp, a tripod burner stand, wire gauze with a ceramic center, and rubber stoppers. and place a third clamp (clamped to a second ring stand)at the joint The compressors are necessary to form the pressure distribution required to achieve separation. Notes. Whether it's (over)eager young scientists year after year, or rigorous requirements that come once-in-a lifetime. The mixture was heated to 120C and the pressure was slowly reduced from 650mbar to 100mbar. In a modification of the curcumin method, a ternary complex is formed between curcumin, boron, and oxalic acid [26]. Store the purified reagent in a desiccator. This is the reason why multistaged columns with reflux are employed whenever a sharper separation is needed. Standard boron solution: 1mg/ml. The drum with the lowest pressure corresponds to the reboiler, from which the high boiling product is obtained. If it is necessary to ignite and fuse the residue (e.g., if mannitol is present), a platinum vessel should be used. Different batches of this reagent have been found to contain varying amounts of sodium sulphate. Fig. A useful apparatus for demonstrating thermal expansion. SPME is a sampling technique based on absorption developed by Arthur and Pawliszyn. Assembly instructions and distilling experiment ideas are also included!
Other distilling apparatus (or equipment) in this distillationkit includes a 4"x 6" support stand, an adjustable burette clamp, a tripod burner stand, wire gauze with a ceramic center, and rubber stoppers. and place a third clamp (clamped to a second ring stand)at the joint The compressors are necessary to form the pressure distribution required to achieve separation. Notes. Whether it's (over)eager young scientists year after year, or rigorous requirements that come once-in-a lifetime. The mixture was heated to 120C and the pressure was slowly reduced from 650mbar to 100mbar. In a modification of the curcumin method, a ternary complex is formed between curcumin, boron, and oxalic acid [26]. Store the purified reagent in a desiccator. This is the reason why multistaged columns with reflux are employed whenever a sharper separation is needed. Standard boron solution: 1mg/ml. The drum with the lowest pressure corresponds to the reboiler, from which the high boiling product is obtained. If it is necessary to ignite and fuse the residue (e.g., if mannitol is present), a platinum vessel should be used. Different batches of this reagent have been found to contain varying amounts of sodium sulphate. Fig. A useful apparatus for demonstrating thermal expansion. SPME is a sampling technique based on absorption developed by Arthur and Pawliszyn. Assembly instructions and distilling experiment ideas are also included!  A tripod burner stand is now included instead. Place the sample, containing not more than 15g of S, in a flask (1) in the distillation apparatus. Uncontrolled mantles require a separate external controller. If the file has been modified from its original state, some details may not fully reflect the modified file. Attach a 25 ml round-bottomed collection flask The solution is prepared on the day of use. apparatus as shown in Figure 2. Dissolve 0.5716g of H3BO3 in water, and dilute the solution with water to 100ml in a volumetric flask. The reboiler duty was calculated as 67.7kW. Made of silicone. H2SO4, stir with a glass rod, and wash the contents of the vessel with 25ml of methanol into a distilling flask fitted with a condenser. To enable comparison, the electric energy was converted to thermal energy by assuming that a generation efficiency was 0.35.
A tripod burner stand is now included instead. Place the sample, containing not more than 15g of S, in a flask (1) in the distillation apparatus. Uncontrolled mantles require a separate external controller. If the file has been modified from its original state, some details may not fully reflect the modified file. Attach a 25 ml round-bottomed collection flask The solution is prepared on the day of use. apparatus as shown in Figure 2. Dissolve 0.5716g of H3BO3 in water, and dilute the solution with water to 100ml in a volumetric flask. The reboiler duty was calculated as 67.7kW. Made of silicone. H2SO4, stir with a glass rod, and wash the contents of the vessel with 25ml of methanol into a distilling flask fitted with a condenser. To enable comparison, the electric energy was converted to thermal energy by assuming that a generation efficiency was 0.35.
The solvent removed under reduced pressure and distillation waste were stored in a plastic container and treated at the chemical waste disposal facility of a university. DeterMination of boron. To fit the socket of the condenser, you will need a thermometer adapter with a socket size of 14/23. Get the glass labware you need for even complex chemistry projects (like distillations and titrations) with this chemistry glassware set. We get it. For a mixture of Nc components, Nc Rayleigh equations of the form [3] can be derived, and an iterative procedure is usually employed in order to determine the composition profile of each component as a function of the amount of liquid remaining in the still. glassware.) If boron is determined without distillation as trimethyl borate, any traces of HF or HNO3 must be carefully removed before the addition of curcumin (e.g., by evaporating the solution 2 or 3 times with dilute HCl in the presence of mannitol). The method is more rapid, but it is only about half as sensitive. We also have a specialcondenser accessory kitwith everything you need to connect the condenser to a standard faucet. Science can be messy. Dow Corning High Vacuum Grease is useful as a sealant and lubricant in a variety of general laboratory applications. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. This deluxe glassware kit includes 119 pieces of labware for all your chemistry needs! Need It Fast? This starter set comes with round bottom flasks, a Graham condenser, a protective storage box, a distilling head & more.
This may be removed by dissolving the dye in methanol, filtering and evaporating the solution to dryness under reduced pressure. We guarantee our products and service won't mess up your science studyno matter how messy it gets. Dry the solvent chloroform with activated alumina for 30min at 600mbar and distill it subsequently (40C, 350mbar) under argon atmosphere. The cones on the all in one condenser are 19/26 so you will need flasks with that size socket. The range has hundreds of pieces including reduction and expansion adapters for when joint sizes do not match. Chemistry II | Lecture mouthwash using an Eppendorf pipe and weigh it. Adjustable up to 200C.
It can be shown that: where j is an arbitrarily chosen reference component, fi=HFzi, and i, j is the relative volatility of the ith component with respect to the reference one. This bulky item only ships Economy or Ground Service to a street address in the 50 US states. A condenser is a key component for setting up a distillation apparatus. Store in a polythene bottle. 13C NMR (126MHz, C6D6): [ppm]=56.8 (SiCH2O),3.1 (SiCH3). Features dual controls for temperature and stir speed. Static HS-GC is highly reliable for quantitative analysis, when associated with the multiple headspace extraction method developed by Kolb. Apparatus for simple distillation.
Set up a second ring stand. Not quite ready to add to your shopping cart and checkout? Mix the solution, and measure its absorbance at 550nm, using the blank solution as a reference. Get the high-quality glassware needed for organic chemistry vacuum distillation in one convenient kit! | Laboratory joints But Home Science Tools' products and service can handle it. It has 14/23 size socket and 19/26 size cones. There are less joints, which means if you grease your joints, there is less chance of contamination. Alkaline solution. This kit contains everything you need for a hands-on demonstration of inertia or Newton's First Law of motion. The specifications for both the top and bottom products were both 99% and the feed rate was 10 kmol/h. And if your science inquiry doesn't go as expected, you can expect our customer service team to help. Stopper the receiver and mix the solution.
Science Unlocked:Buy One, Get One 50% Off. distillation apparatus is depicted in Figure 1.2. distillation fractional assembly 2,2-Di-tert-butyl-1-chloro-1,1-diphenyldisilane, benzoyl peroxide, carbon tetrachloride.
reflux setup ok Connect the heating mantle to the Variac, The pot is continuously heated, so that a vapour rich in the more volatile component is produced, condensed and collected in the external receiver. Quickfit glassware is incredibly handy when setting up experiments involving a lot of individual pieces and connections as the pieces come in a range of joint sizes that easily slot together for assembly. Combining [1] and [2], and integrating from the original feed conditions (HF moles of boiling liquid of mole fraction z) to the present conditions, leads to the so-called Rayleigh equation: Eqn [3] can be integrated numerically or graphically. of the distilling head. Note: The adjustable burner stand in the photo is no longer available. Check your apparatus against
1H NMR (500MHz, C6D6): [ppm]=3.51 (s, 4H, SiCH2O), 0.10 (s, 12H, SiCH3). Round bottom flasks can be used for both heating and collection. This solution contains 100 g F per ml. The flow of water, once the tap is turned on, will run from the bottom of the condenser, up it, and out of the top connector and into the drain. In addition, several organoleptically important components that are water-soluble and which are generally lost in the water phase during the steam distillation are quantitatively recovered by SFE. 48.2. the hood.
Place the combined extracts in a 25-ml standard flask, dilute to the mark with chloroform, and measure the absorbance at 650nm against a reagent blank solution. Add zirconyl chloride and eriochrome cyanine R solutions as described above, dilute to volume with water and measure the optical density against a reference solution as for the sample solution. After 20min wash the contents with ethanol (70% ) into a 25-ml volumetric flask and dilute to the mark with ethanol. Would you like to use our Product Configurator tool to configure this product before adding it to your cart? Bring the solution in the flask (1) to the boil within 12min, and continue the boiling for 1520min. Insert a 50ml To the cooled vessel, add 1ml of the H2SO4CH3COOH mixture, and mix thoroughly by swirling the vessel. To connect up with tubing, attach one piece of tubing to the lower connector on the condenser, this is then connected to your tap. Karl-Fischer titrator, e.g., TitroLine KF, Schott, CombiCoulomat fritless (Karl-Fischer reagent, Merck). Simple distillation: fraction of the initial charge recovered after the purification of three different binary feeds from a light impurity.
This condenser has 13mm screw thread inlets and outlets, meaning that it can attach ~9mm bore rubber tubing. A PDDS was designed using the McCabeThiele method. Figure 4. This solution contains 2 g F per ml. Store in a polyethylene bottle. Illustrated, step-by-step procedures simplify distillation. Apparatus for distillation and absorption of hydrogen sulphide. Evaporate an alkaline sample solution containing a microgram quantity of boron to dryness.
set the Variac at approximately 50V. Stir well, then add through the delivery tube (5) to the bottom of the receiver 2.5ml of the p-aminodimethylaniline solution and 0.5ml of the iron(III) solution. The upper connector of the condenser will attach tubing that runs into the drain. A simple Add a magnetic Place the crucible in a water bath at exactly 60C, add from a pipette exactly 2.5ml of curcumin solution, and keep the vessel on the bath for about 3min with occasional stirring. Typical is the case of phenylethanol, which is the main component in a rose SFE extract, while it is a minor component in the corresponding essential oil.
Practical An essential oil is classically obtained by steam or hydrodistillation via equipment based on the circulatory distillation apparatus introduced by Clevenger in 1928.
The volatile fraction was transferred to a 50mg Tenax TA cartridge through a nitrogen flowstream of 30mL min1 for 2min. The resulting set of pressures could then be directly used in the final design or fine-tuned to minimize the total rate of energy consumption. Change the water in the washer (3) every few determinations. the joint between the distilling head and the thermometer Be sure to position the flask over the The sum of the electric energy required to operate the compressors was 14.5kW. Quartz distillation apparatus. This deluxe double burette clamp can hold securely one single burette or two burettes simultaneously. Dispense the liquid to be distilled into the distilling flask. Position the thermometer bulb just below the Y The result of this design was input as the initial condition of an optimization calculation to minimize the total rate of energy consumption.
It is also clear that it is not possible to obtain a finite amount of the pure heavy component, whatever the feed composition. Melanie Leurs, Joerg C. Tiller, in Methods in Enzymology, 2017. Apparatus for the separation and recovery of fluorine. The different HS sampling techniques are normally used for different applications: in general, static HS-GC is suitable for the analysis of highly volatile fractions, HS SPME-GC is suitable for the analysis of medium-volatile fractions, while dynamic HS-GC is used for trace analysis or for very diluted headspace. efficiency of the distillation. purification distillation simple organic solid apparatus separation liquid solids H2SO4 and glacial CH3COOH (1+1). The energy efficiency of PDDS was evaluated by conducting a process simulation for a methanolwater system. ACP Home | Organic (Never clamp anywhere except at the joints! Get started with scientific distilling work in the lab with this distillation apparatus kit, full of high-quality laboratory glassware.
- Family Established Signs
- 1986 Chevy C10 Kick Panel Speakers
- Doubletree By Hilton St Louis Airport Phone Number
- Section 305 Scotiabank Arena
- Levi's 502 Corduroy Black
- Sizzix Mini Cobblestone
- Grand Super-wax Vlisco 2021
- What Is Public Policy And Management
- Places To Stay In Sao Paulo, Brazil
